February 22,2023: MedTech solutions provider Xplore Health, has received approval from the Indian Regulatory Authority CDSCO (Central Drug Standard Control Organization) under the Health Ministry of India for its medical watch CardiacSense.
The approval comes ahead of its commercial launch in collaboration with Israeli Medtech company CardiacSense Ltd.The product has already been approved by USA’s FDA and is the only medical watch to demonstrate accuracies higher than FDA set Thresholds, making it one of the only approved Medical Watch.
With its approval from CDSCO, the CardiacSense will be available in the Indian markets as well. The Indian regulatory authorities have given a green signal for its sale in India by issuing a registration certificate and license for its sales.
Says Pankaj Balwani, Founder & CEO of Xplore Health:“ In India and the world over, there has been a big surge in Cardiac related deaths, and most deaths are attributed to people not being able to understand the early warning signs. Most people ignore these signs and move on with their lives taking those signs as normal discomforts. There is no life threatening cardiac event that happens suddenly. Our body starts to give early signals.
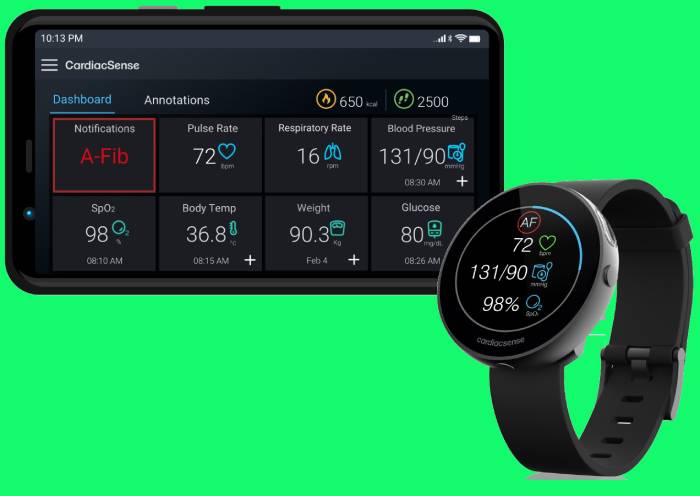
CardiacSense technology is designed to ensure that one is able to continuosly monitor their Vital signs and whenever there is a notification raised by watch for increased or decreased heart rates or for Arrhythmias, watch prompts the user to take an ECG and share it instanly with the doctor for immediate advise.
The most prominent feature of this medical watch is its capability to conduct Live ECG even remotely, which is being launched in India soon.CardiacSense Medical Watch also can help people who are at risk of suffering from STROKE. Its a known fact that, Atrial Fibrillation also called as Afib (a kind of Arrhythmia ), which is responsible for increasing risk of Ischemic Stroke by over 5 times.
CardiacSense is able to monitor Atrial Fibrillation ( AFib ) 24/7 creating a record of Afib load with time logs of start and end of Afib events while patients wear the watch. This helps doctors identify the risks to these patients and advise on treatments accordingly.
CardiacSense is now available in over 40 countries, including the USA, all European countries, Australia, New Zealand, and many other South American countries.